The Meals and Drug Administration (FDA) has granted a breakthrough remedy designation to be used of the positron emission tomography (PET) imaging agent 124I-evuzamitide (AT-01) for sufferers with suspected or recognized cardiac amyloidosis.
The primary non-invasive pan-amyloid PET agent designed for systemic amyloidosis, 124I-evuzamitide has reportedly demonstrated effectiveness in detecting a number of forms of amyloid deposits within the liver, kidney, and coronary heart, in keeping with Attralus, the developer of 124I-evuzamitide.1
The primary non-invasive pan-amyloid PET agent designed for systemic amyloidosis, 124I-evuzamitide just lately garnered a breakthrough remedy designation from the FDA to be used in sufferers with suspected or recognized cardiac amyloidosis. (Photos courtesy of Attralus.)
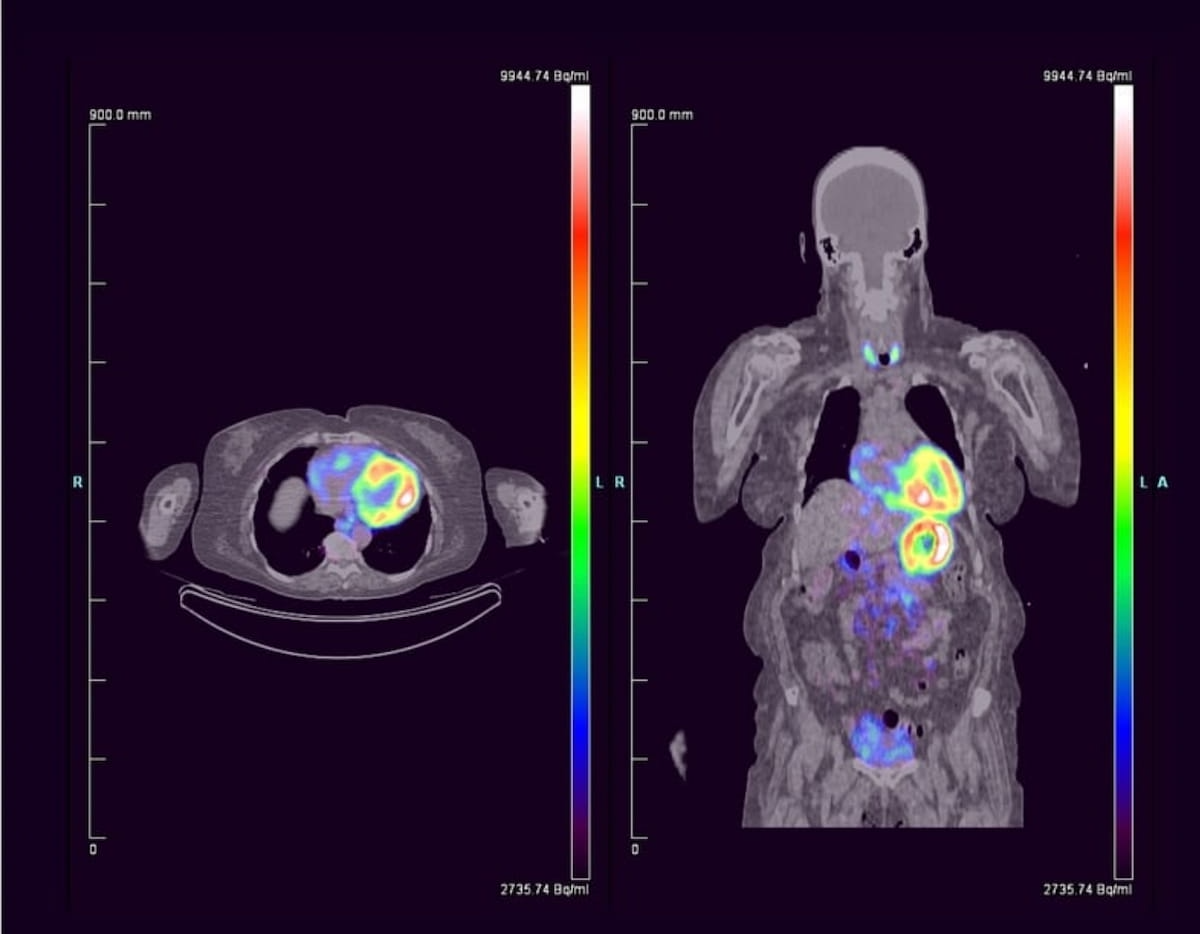
In a latest research evaluating the usage of 124I-evuzamitide for 50 sufferers with systemic amyloidosis, researchers famous a 96.2 p.c cardiac-associated sensitivity price and one drug-related antagonistic occasion.2
Emphasizing that 124I-evuzamitide is the one diagnostic imaging agent to garner a breakthrough remedy designation for cardiac amyloidosis, Attralus says the FDA’s resolution was primarily based on a number of research evaluating use of the radiotracer in sufferers with cardiac amyloidosis.1
“We’re extremely inspired by FDA’s resolution to grant Breakthrough Remedy Designation to 124I-evuzamitide (AT-01), recognizing its potential as an revolutionary diagnostic agent for sufferers with systemic amyloidosis” stated Gregory Bell, M.D., the chief medical officer at Attralus. “There are not any FDA accredited diagnostic imaging brokers for cardiac amyloidosis. The analysis of cardiac amyloidosis is a difficult and time-consuming course of for sufferers, with many going years with out an correct analysis, and shedding vital time within the course of.”
Attralus added that 124I-evuzamitide (AT-01) would be the topic of a part 3 research of sufferers with suspected cardiac amyloidosis that’s slated to start in 2025.
References
1. Attralus. Attralus receives breakthrough remedy designation for its pan-amyloid diagnostic PET imaging candidate 124I-evuzamitide (AT-01) for cardiac amyloidosis. Globe Newswire. Out there at: https://www.globenewswire.com/news-release/2024/08/05/2924179/0/en/Attralus-Receives-Breakthrough-Remedy-Designation-for-its-Pan-Amyloid-Diagnostic-PET-Imaging-Candidate-124I-evuzamitide-AT-01-for-Cardiac-Amyloidosis.html . Revealed August 5, 2024. Accessed August 5, 2024.
2. Wall JS, Martin EB, Lands R, et al. Cardiac amyloid detection by PET/CT imaging of iodine (124I) evuzamitide (124I-p5+14): a part 1/2 research. JACC Cardiovasc Imaging. 2023;16(11):1433-1448.

