That includes quite a lot of synthetic intelligence (AI) instruments to boost breast most cancers detection with digital breast tomosynthesis (DBT), the SmartMammo Dx software program has garnered expanded clearance from the Meals and Drug Administration (FDA).1
Beforehand cleared by the FDA in 2021 to be used with Hologic mammography techniques, SmartMammo Dx (DeepHealth/RadNet) can now be used with Senographe Pristina (GE HealthCare) mammography platforms.
Assigning finding- and case-specific suspicion ranges for breast most cancers based mostly on identification of sentimental tissue lesions and calcifications on digital breast tomosynthesis (DBT), the SmartMammo Dx software program lately garnered expanded FDA clearance to be used with the Senographe Pristina (GE HealthCare) mammography platforms. (Picture courtesy of DeepHealth.)
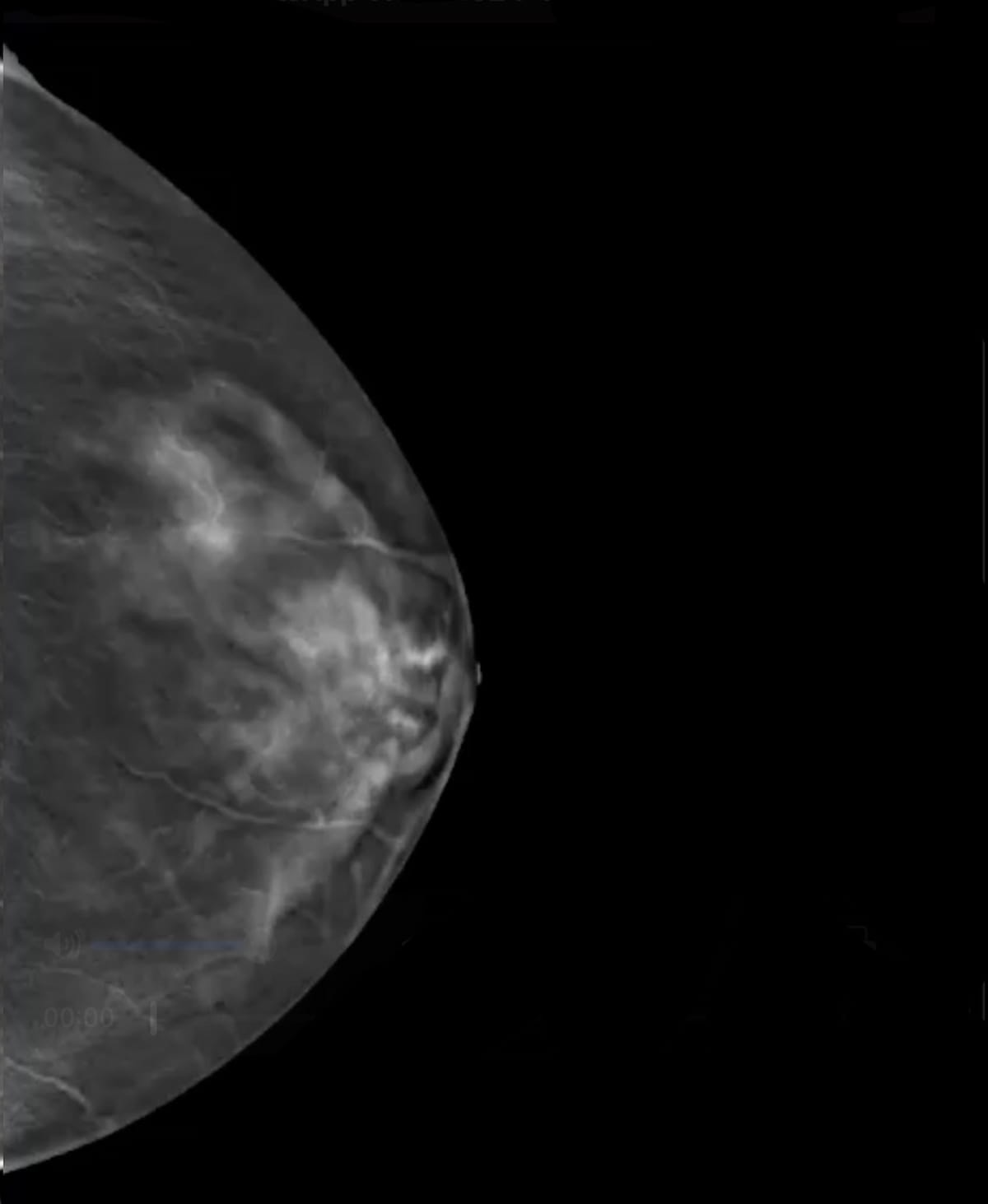
DeepHealth stated the SmartMammo Dx software program assigns finding- and case-specific suspicion ranges based mostly on identification of sentimental tissue lesions and calcifications on DBT exams. The SmartMammo software program has facilitated 23 % increased detection of breast most cancers in ladies with dense breasts and a 20 % increased detection of breast most cancers detection in African American ladies, based on DeepHealth.2
“This FDA clearance permits us to increase entry to high-quality breast most cancers screening to extra sufferers,” stated Kees Wesdorp, the president and CEO of DeepHealth. “By bringing SmartMammo’s improved most cancers detection to GE HealthCare’s mammography techniques, extra suppliers can harness the facility of AI to assist redefine radiology workflows and deal with key challenges throughout the imaging worth chain, to enhance velocity, medical accuracy, operational effectivity, and elevate affected person care.”
References
1. RadNet. Correction: RadNet’s DeepHealth subsidiary expands FDA clearance for SmartMammo™ resolution. GlobeNewswire. Accessible at: https://www.globenewswire.com/news-release/2024/11/29/2989189/0/en/CORRECTION-RadNet-s-DeepHealth-Subsidiary-Expands-FDA-Clearance-for-SmartMammo-Answer.html . Printed November 29, 2024. Accessed November 29, 2024.
2. DeepHealth. SmartMammo. Accessible at: https://deephealth.com/population-health/smart-mammo/ . Accessed November 29, 2024.

