The Meals and Drug Administration has granted 510(ok) clearance for the Max 3™ distinction media injector to be used in magnetic resonance imaging (MRI) exams.
Providing ease of use options to foster workflow efficiencies, the Max 3 is a syringe-less injector, in accordance with Bracco Diagnostics, the producer of the modality, which was developed in collaboration with Ulrich Medical.
The syringe-less distinction media injector Max 3 garnered 510(ok) clearance from the Meals and Drug Administration (FDA) to be used in magnetic resonance imaging (MRI) exams. (Picture courtesy of Bracco Diagnostics.)
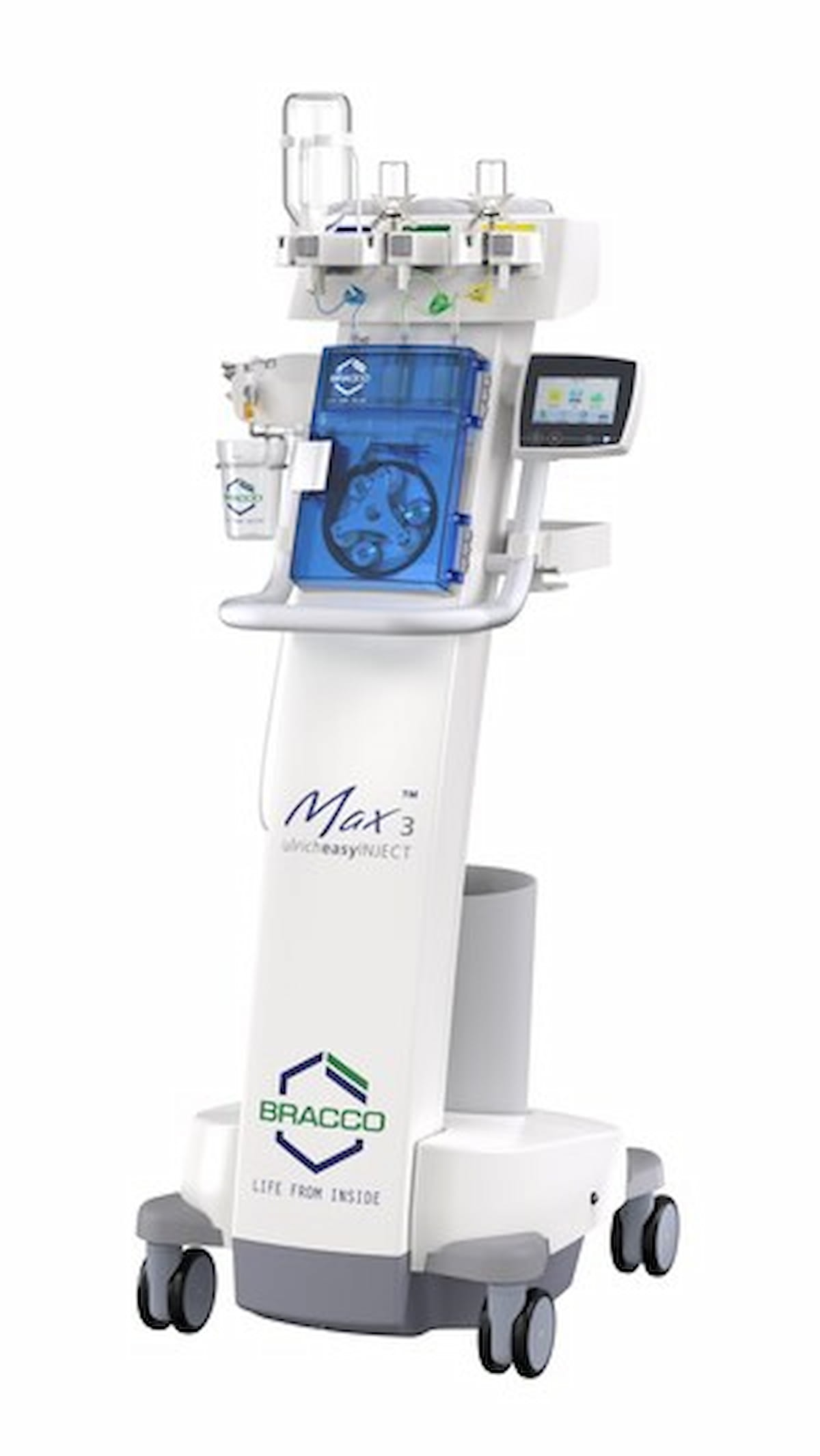
Emphasizing direct injection from unique distinction media vials, Bracco Diagnostics highlighted the Max 3’s mixture of the Straightforward-Click on Cassette flex and SafeConnect. The corporate famous these options allow devoted connection to affected person tubing with safety from retrograde contamination. The connector with the Straightforward-Click on Cassette will be utilized inside a 24-hour interval or as much as a most of 96 bottles of distinction media, whichever happens first, in accordance with Bracco Diagnostics.
The corporate identified that the dearth of energy cables with Max 3 allow handy positioning of the gadget throughout the MRI room. Along with eliminating the necessity to refill syringes, Bracco Diagnostics stated use of the Max 3 reduces disposable plastic waste.
“We’re thrilled to deliver an intuitive, easy-to-use, and environmentally pleasant answer to the radiology group in collaboration with Bracco,” stated Klaus Kiesel, the chief government officer of Ulrich Medical. “Collectively, we’re taking MRI innovation to the subsequent degree in well being care.”

