The Meals and Drug Administration (FDA) has granted 510(ok) clearance for PlaqueIQ™, an rising software program that will improve quantification of arterial plaque buildup proven on coronary computed tomography angiography (CCTA) imaging.
Using picture restoration algorithms to scale back movement and calcium blooming artifacts, the Plaque IQ software program creates 3D modelling of coronary arteries through knowledge segmentation and subsequently supplies quantified evaluation of tissue composition and construction, in keeping with Elucid, the developer of PlaqueIQ.
Providing an interactive visualization of coronary anatomy and plaque quantification on a number of views, the PlaqueID software program not too long ago garnered 510(ok) clearance from the Meals and Drug Administration (FDA). (Photos courtesy of Elucid.)
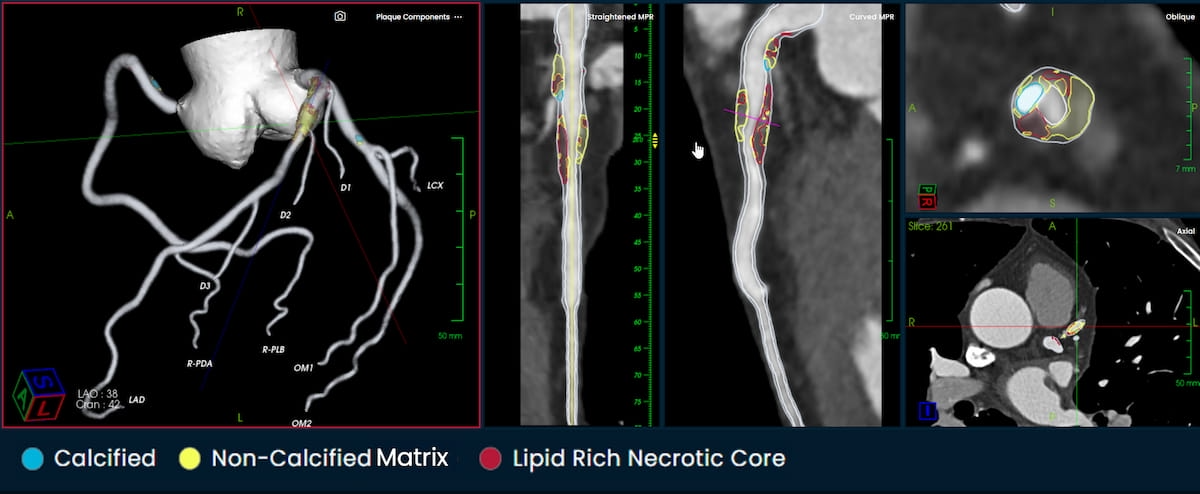
Emphasizing that roughly half of the United State inhabitants between 45 to 84 years of age have atherosclerosis with out symptomatic displays, Elucid maintained that PlaqueIQ might facilitate enhanced visualization and characterization of arterial plaque, and prevention of main adversarial cardiovascular occasions.
“It’s time to shift our focus from merely estimating danger and treating danger of MI to immediately visualizing and treating the illness itself by trying on the coronary arteries,” maintained Amir Ahmadi, M.D., an affiliate professor of medication and cardiology on the Icahn College of Medication at Mount Sinai in New York, N.Y. “I consider that PlaqueIQ will allow physicians to raised ‘see’ the illness — particularly plaque amount and kind — in order that we are able to deal with sufferers with higher precision and in (a) personalised method, enhance their high quality of life, and in the end stop (myocardial infarction) and stroke extra successfully.”

