The Meals and Drug Administration (FDA) has granted 510(okay) clearance to the Medrad Centargo CT Injection System, which allows distinction administration for a number of sufferers present process computed tomography (CT) exams.
That includes a pre-assembled Day Set and a snap-in affected person line that auto-primes upon insertion, the Medrad Centargo CT Injection System permits distinction set-up for a number of sufferers to be accomplished in lower than two minutes, in accordance with Bayer, the producer of the Medrad Centargo CT Injection System.
The newly FDA-cleared Medrad Centargo CT Injection System reportedly permits distinction set-up for a number of sufferers to be accomplished in lower than two minutes. (Picture courtesy of Bayer.)
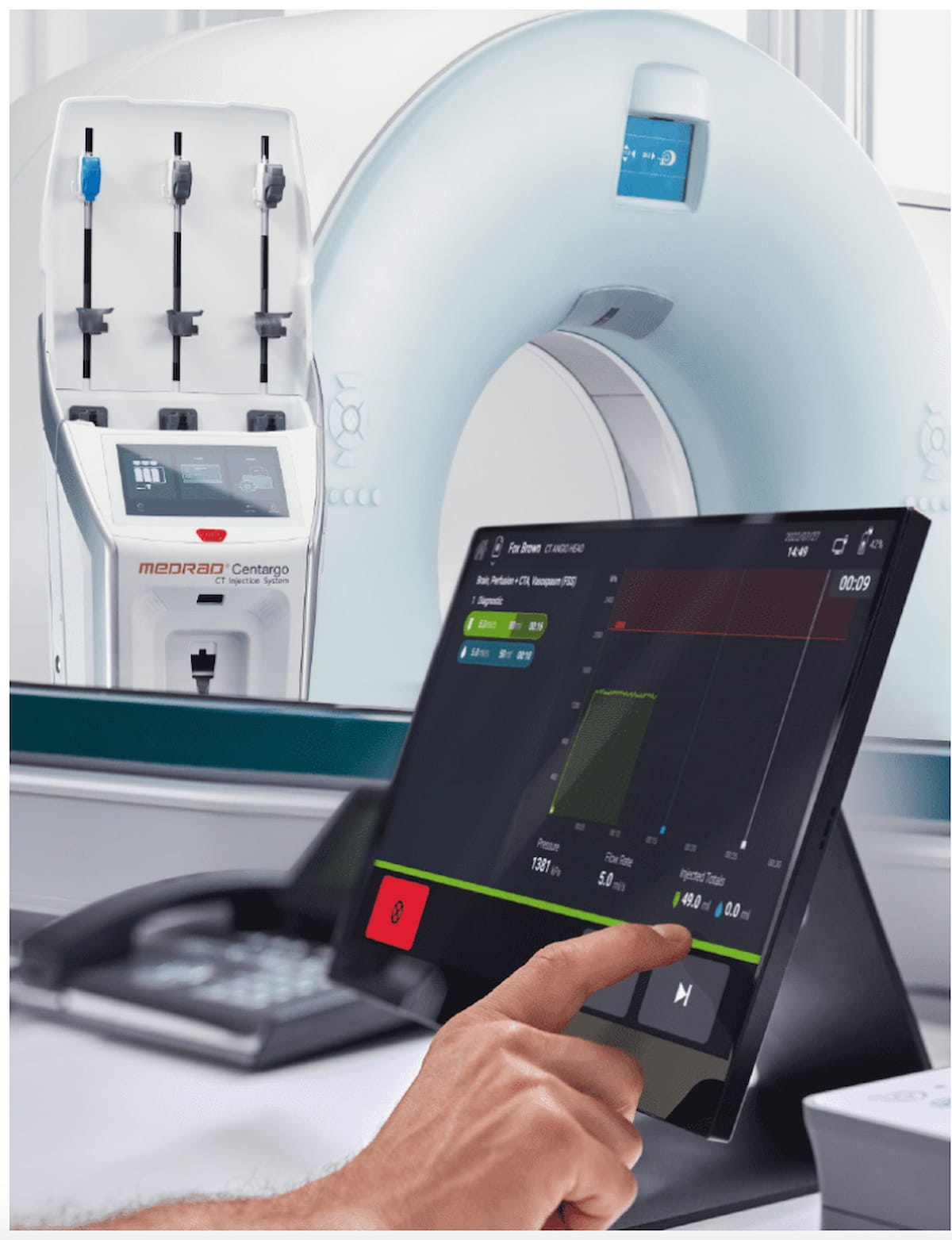
For demanding CT protocols, Bayer stated the system additionally has a Twin Stream function that enables simultaneous administration of distinction and saline, which is facilitated by the Medrad Centargo CT Injection System’s piston-based expertise.
The mix of automated documentation software program and the system’s built-in barcode reader allows simplified entry to distinction and injection particulars to assist streamline reporting.
“We all know radiologists in the present day are going through a repeatedly growing workload, and the automation, integration and mobility of the Medrad® Centargo CT Injection System helps radiology workers do extra with much less, permitting them extra time to concentrate on their sufferers,” famous Sven Schmidt, the top of Area Americas Radiology at Bayer.

