The Meals and Drug Administration (FDA) has granted its breakthrough system designation and 510(ok) clearance for AutoChamber™, a synthetic intelligence (AI)-enabled software program for computed tomography (CT) that will facilitate early detection of enlarged coronary heart chambers.
Leveraging deep learning-based AI, the AutoChamber software program calculates the quantity of cardiac chambers and left ventricular wall mass in 15 to twenty seconds, in response to HeartLung Applied sciences, the producer of AutoChamber.
The newly FDA-cleared AutoChamber software program leverages deep learning-based AI to calculate the quantity of cardiac chambers and left ventricular wall mass in 15 to twenty seconds, in response to HeartLung Applied sciences, the developer of the software program. (Pictures courtesy of HeartLung Applied sciences.)
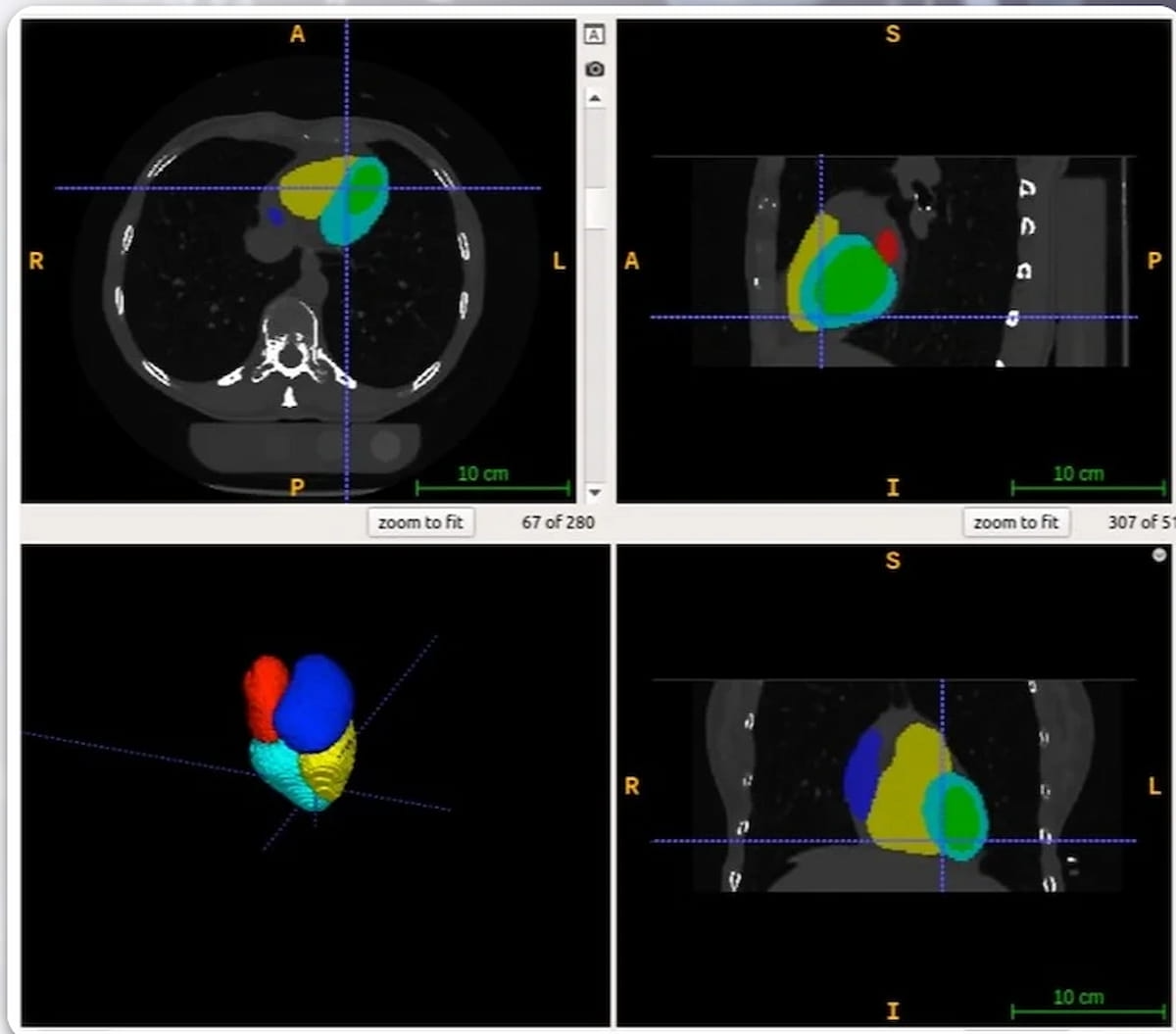
The corporate mentioned AutoChamber, which will be utilized for coronary heart CT and coronary CT angiography (CCTA) scans in addition to chest CT for lung most cancers screening, might assist determine sufferers who’ve increased dangers for atrial fibrillation, stroke, and coronary heart failure.
“I really feel strongly in regards to the alternative AutoChamber might have to guage cardiovascular danger for the hundreds of thousands of individuals getting chest CT scans worldwide, particularly for lung most cancers screening and different non-cardiac causes,” famous Nathan Wong, M.D., a professor of director of the Coronary heart Illness Prevention Program inside the Division of Cardiology on the College of California, Irvine in Irvine, Calif. “The potential is for figuring out these at elevated danger who may benefit from earlier intervention to enhance outcomes.”

