The Meals and Drug Administration (FDA) has granted 510(okay) clearance for the Signa Magnus system, a 3T magnetic resonance imaging (MRI) system for head-only scanning.
That includes HyperG gradient know-how, the Signa Magnus system facilitates sooner MRI scanning with efficiency ranges of 300 mT/m and 750 T/m/s, based on GE HealthCare, the producer of the system. Along with shorter scan occasions, the corporate mentioned the neuroimaging system gives superior diffusion imaging capabilities and a excessive signal-to-noise ratio (SNR).
GE HealthCare mentioned the head-only emphasis of the newly FDA-cleared Signa Magnus 3T MRI system facilitates a gradient amplitude and slew charge that exceed these of typical whole-body MRI platforms. (Picture courtesy of GE HealthCare.)
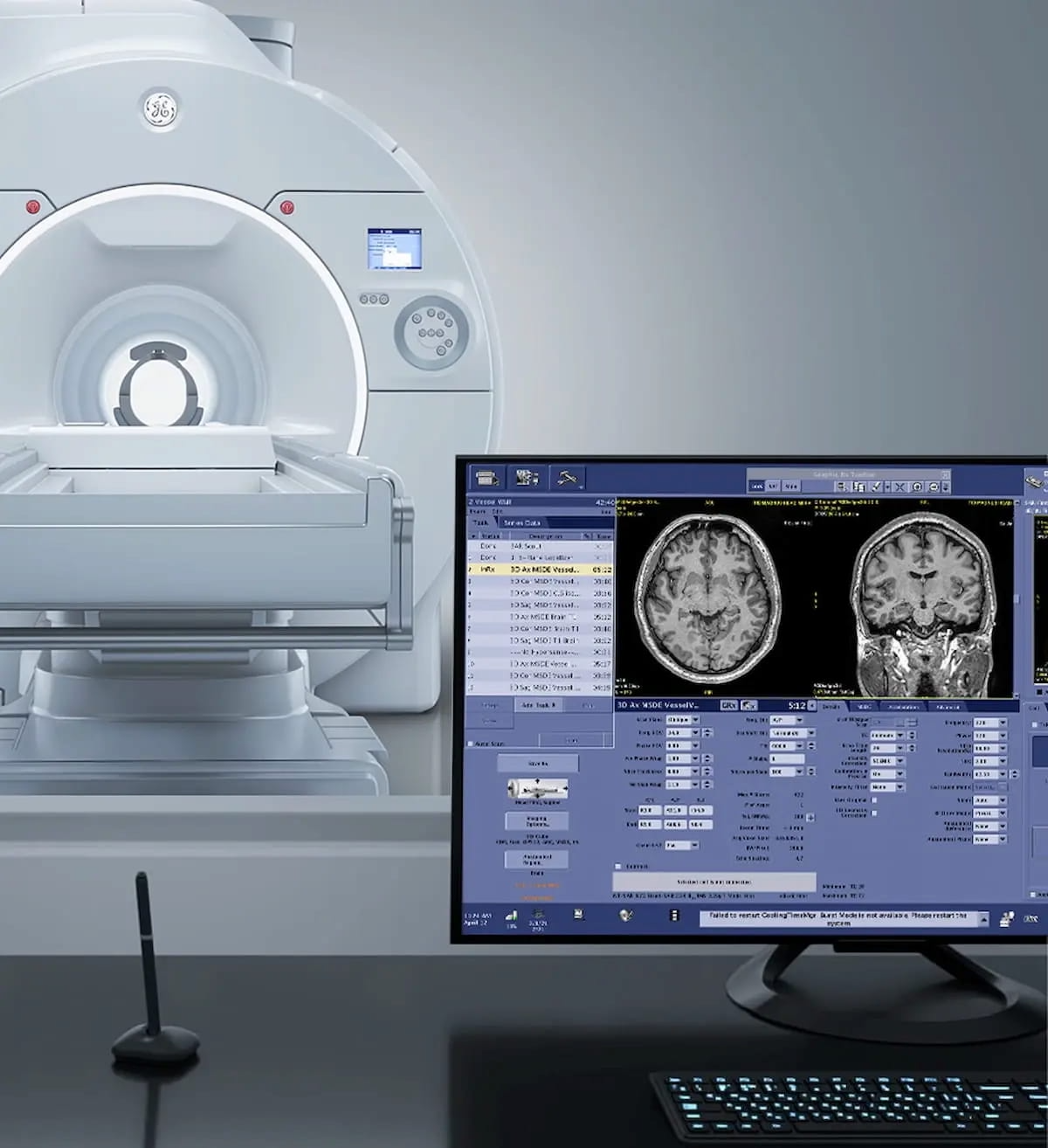
Noting an asymmetrical design that shifts the gradient isocenter to the affected person fringe of the coil, GE HealthCare mentioned the head-only emphasis of the system facilitates a gradient amplitude and slew charge that exceed these of typical whole-body MRI platforms.
“We’re very excited concerning the capabilities Signa Magnus gives,” mentioned Kawin Setsompop, Ph.D., an affiliate professor of radiology at Stanford College. “I plan to leverage the gradient efficiency to take a look at microstructures with diffusion imaging, comparable to axonal diameter. Moreover, utilizing the excessive slew charge for environment friendly readout by way of EPI and spiral to pattern k-space sooner will assist obtain greater decision with fewer artifacts.”

